RESEARCH
The overall focus of our work is to target the morphogen Nodal in aggressive cancers, blocking its interaction with the receptor Cripto-1 — using a novel, first-in-class antibody drug.
NODAL
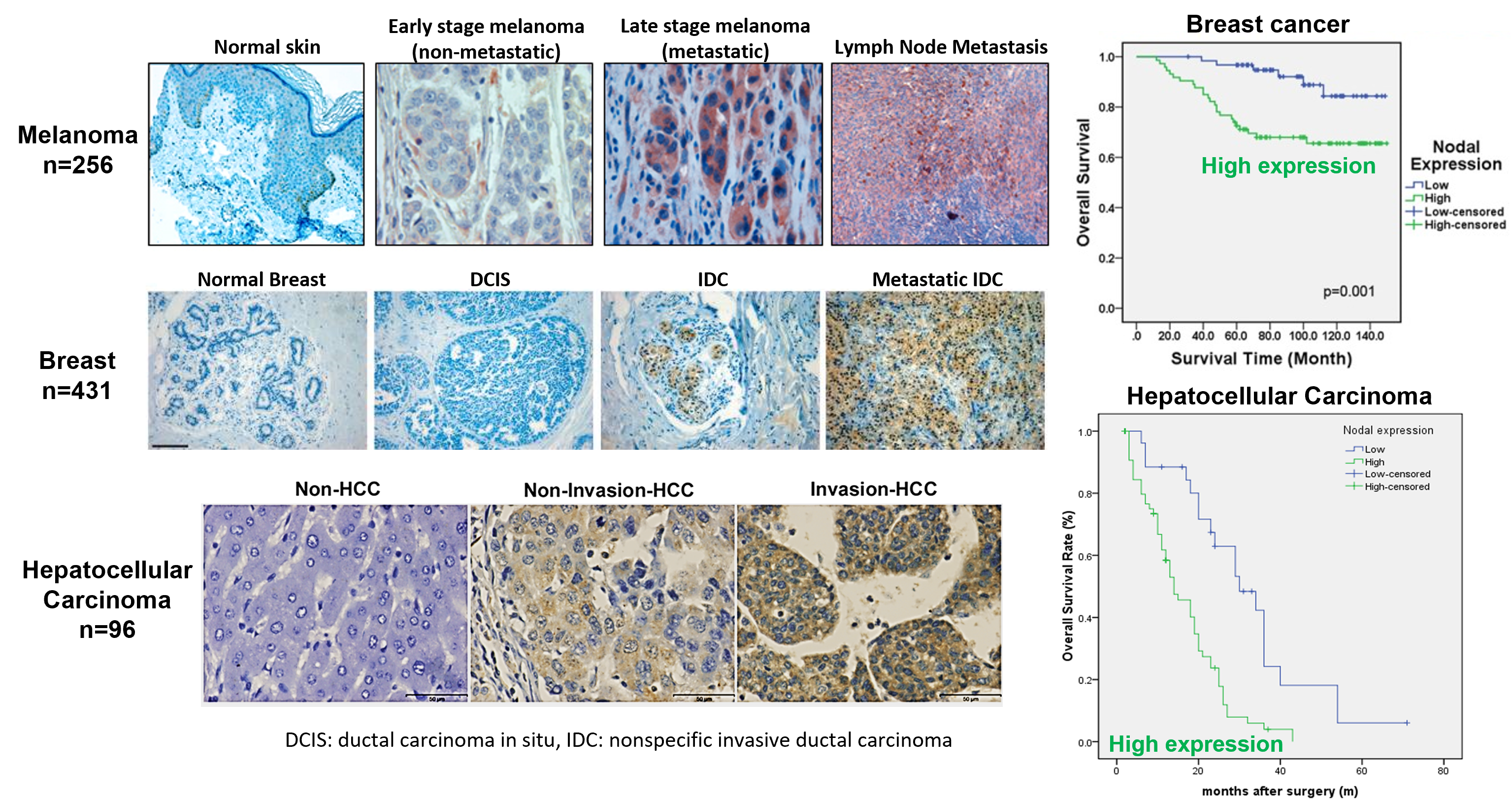
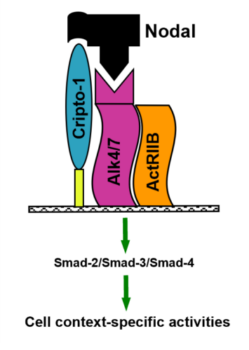
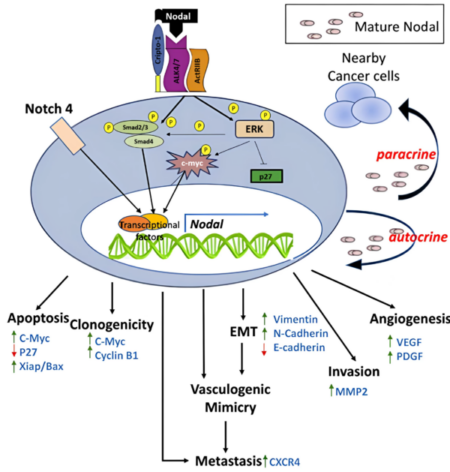
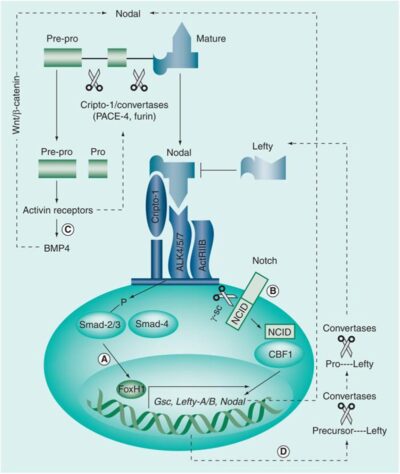
Nodal, a morphogen belonging to the TGF-β superfamily, plays a critical role in embryonic stem cell signaling. It is essential for embryonic axis formation and Left/Right patterning during early development. While Nodal expression is largely lost in most adult tissues, it becomes reactivated in aggressive tumors. Its re-emergence and elevated expression have been associated with poor prognosis and tumor progression in many cancers, such as melanoma, breast cancer, pancreatic cancer, and hepatocellular carcinoma (Fig. 1) – to name a few. Cripto-1 functions as a co-receptor for Nodal and induces the formation of a heteromeric receptor complex with ALK4/7 (ActRIB) and ActRIIB. This complex initiates downstream signaling by activating Smad-2/3 proteins through phosphorylation. The phosphorylated Smad-2/3 proteins then associate with Smad-4, translocating to the nucleus to activate the transcription of Nodal target genes and subsequently mediate cell-context-specific responses (Fig. 2). Additionally, Notch 4 signaling can upregulate Nodal and other Nodal-responsive genes, reinforcing the pathway. Nodal signaling is thus capable of autocrine and paracrine activation—secreted Nodal can stimulate neighboring cancer cells, further amplifying its signaling cascade. In cancer cells, reactivation of Nodal signaling is often linked to malignant phenotypes. It drives transcriptional programs that support apoptosis, clonogenicity, vasculogenic mimicry, epithelial-mesenchymal transition (EMT), invasion, metastasis, and angiogenesis (Fig. 3).
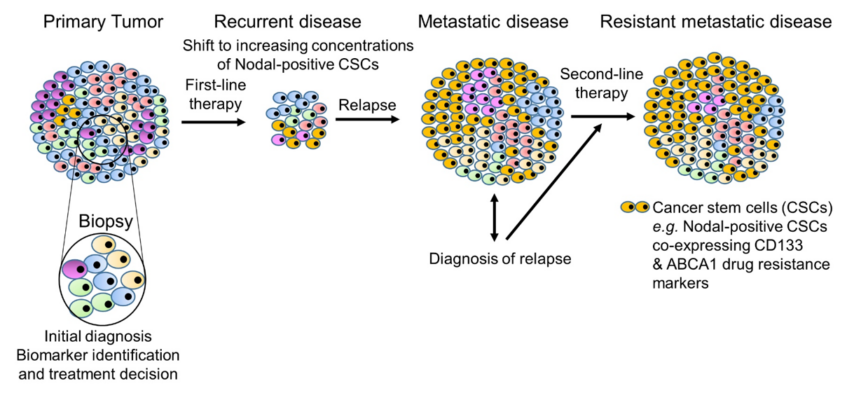
Nodal signaling is a key driver of self-renewal, maintaining stemness and plasticity in cancer stem cells (CSCs)—a minority subpopulation within heterogeneous tumors. These CSCs commonly express drug resistance markers alongside elevated Nodal levels (Fig. 4). While conventional therapies can effectively reduce the bulk tumor population, they often fail to eradicate CSCs, leading to tumor relapse and progression to metastasis.
Our studies have demonstrated that both Nodal and the drug resistance transporter ABCA1 are downregulated following treatment with a neutralizing anti-Nodal antibody (WS65, Fig. 5A). Conversely, post-treatment analysis of breast cancer patient samples revealed increased expression of Nodal and ABCA1, suggesting therapy-induced enrichment of the CSC population (Fig. 5B and 5C).
Furthermore, we found that anti-Nodal antibody used in a combinatorial approach exhibits a more lethal effect on cancer cells. For example, anti-Nodal antibody synergizes with Dabrafenib to inhibit tumor growth in advanced melanoma harboring the BRAF V600E mutation—a setting typically associated with resistance to BRAF inhibitor (BRAFi) monotherapy (Fig. 6). In melanoma and breast cancer models, these findings suggest that Nodal expression drives CSC phenotypes and contributes to therapeutic resistance, at least in part through ABCA1-mediated mechanisms. Blocking Nodal signaling can thus overcome drug resistance and suppress tumor aggressiveness.
Collectively, our results position Nodal as both a prognostic biomarker and a promising therapeutic target in cancer, particularly in patients with refractory or relapsed disease. We are refining a novel neutralizing anti-Nodal antibody (TRX-NOC) that disrupts Nodal–Cripto-1 binding, thereby blocking downstream signaling and attenuating tumor progression, metastasis, and vasculogenic mimicry. TRX-NOC will be a first-in-class anti-Nodal antibody for treating a variety of cancer types. As a secreted protein, Nodal is detectable in the serum of cancer patients. To support personalized therapy, we are also developing a companion diagnostic kit to measure circulating Nodal levels, facilitating patient selection for anti-Nodal antibody treatment.
| Drug Candidate | TRX-NOC Ab |
|---|---|
| IgG class | Humanized IgG1 |
| Target | Human Nodal (Soluble protein) |
| Epitope | hNodal pre-helix loop (44-56) |
| Expected Affinity (KD) | nM level |
| Proposed MoA |
|
| Fc effector function | N/A |
LEFTY
In parallel with our work on Nodal, we are actively investigating Lefty, a naturally occurring antagonist of the Nodal signaling pathway. Lefty exerts its inhibitory function by binding directly to Nodal or its co-receptor Cripto-1, thereby preventing the formation of the signaling complex and subsequent activation of downstream pathways (Fig. 7).
Our studies have demonstrated that human embryonic stem cell (hESC)-derived Lefty significantly suppresses melanoma cell proliferation and promotes apoptosis in vivo (Fig. 8). These findings highlight Lefty’s potential as a biologically inspired therapeutic agent capable of counteracting Nodal-driven tumor progression.
Building on this, we are developing a functional recombinant form of Lefty designed for topical application in the treatment of melanoma and dysplastic nevi. This approach offers a localized, non-invasive therapeutic option that leverages the tumor-suppressive properties of endogenous Nodal pathway inhibitors.
To date, there are no approved therapies specifically targeting the Nodal–Lefty signaling axis. Our development of a neutralizing anti-Nodal antibody and recombinant Lefty protein represents a novel, first-in-class strategy aimed at overcoming therapeutic resistance and providing new treatment options for patients with advanced or refractory cancers. We also have published experimental data demonstrating the promise of blocking Nodal mRNA resulting in significantly diminished tumorigenesis.
Fig. 1 Nodal re-emergence occurs in many types of aggressive cancer
Ref: Postovit et al., PNAS 2008;105(11):4329–4334; Strizzi et al., Breast Cancer Res 2012;14(3):R75; Yu et al., Mod Pathol 2010;23(9):1209-14; Gong et al., Am J Cancer Res 2017;7(3):503-517; Chen et al., PLoS One 2014;9(1):e85840.
Fig. 2 Nodal binds to its heterocomplex receptor to stimulate the Smad 2/3 signaling pathway
Fig. 3 Nodal signaling underlies the cancer stem cell phenotype, unregulated tumor growth and metastasis, and resistance to conventional therapies
Fig. 4 Nodal expression drives the cancer stem cell phenotype and resistance to conventional therapies
Ref: Hendrix et al., In book: Cutaneous Melanoma: Etiology and Therapy. Brisbane (AU): 2017. Chapter 4 (pp.57-65)
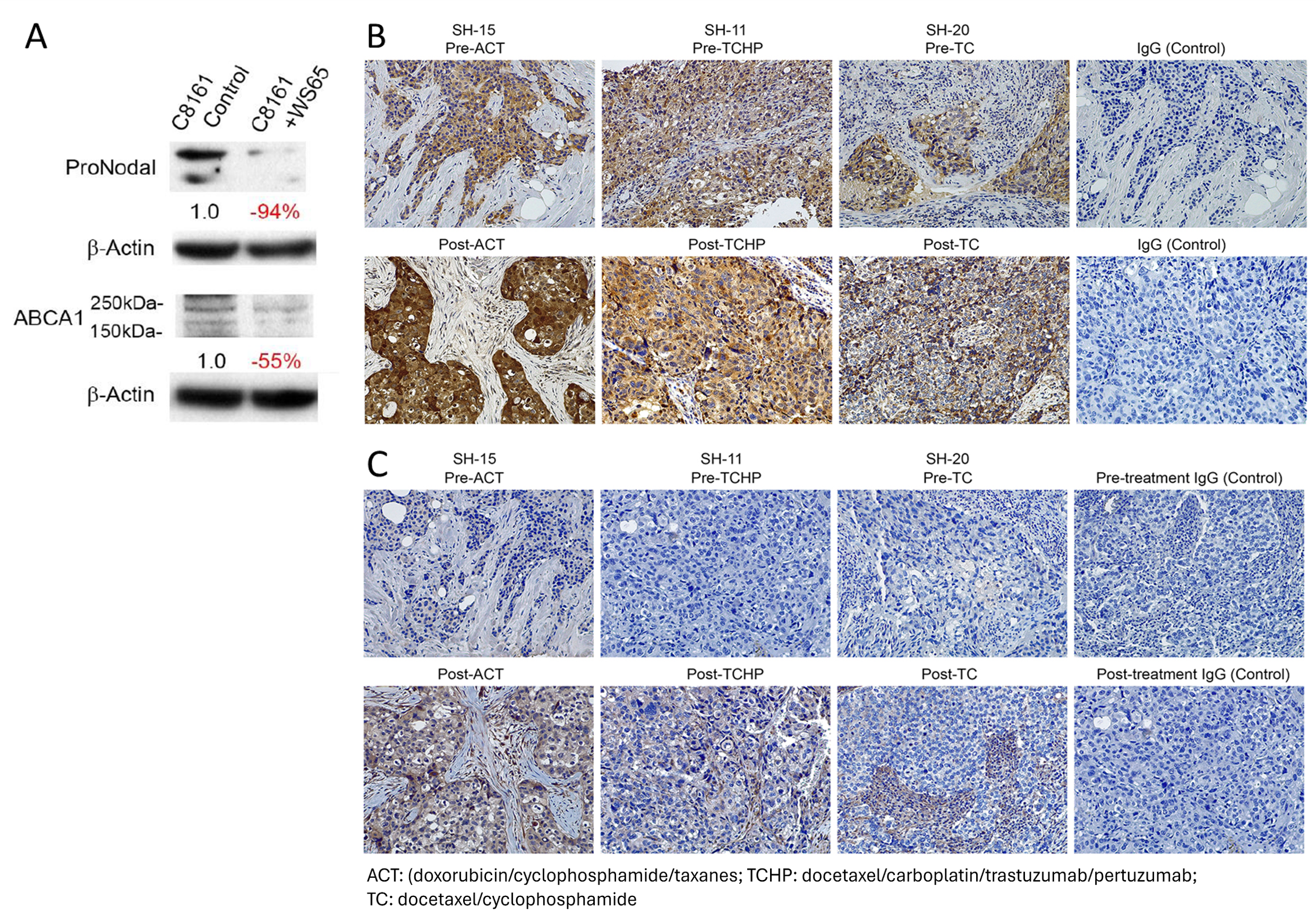
Fig. 5 The association of Nodal and ABCA1 expression are concomitant with chemoresistance in tumors
(A) Western blot analysis of Nodal and ABCA1 (ATP-binding cassette transporter A1) protein level in C8161 melanoma cells treating with WS65 (anti-Nodal antibody) for 72 hrs. The reduction percentage in protein level was shown. (B) The presence of the Nodal protein (brown color) in breast cancer patient tumor sections, pre- and post-current standard-of-care-treatments (ACT, TCHP and TC), was examined by immunohistochemical staining with IgG used as a control for non-specific staining (X20 original magnification). (C) The presence of the ABCA1 protein (brown color) in breast cancer patient tumor sections pre- and post-current standard-of-care-treatments (ACT, TCHP and TC) was examined by immunohistochemical staining with IgG used as a control for non-specific staining (X20 original magnification)
Ref. Seftor et al., Adv Exp Med Biol. 2019; 1139:105-114; Margaryan et al., Cancers 2019; 11(3):340.
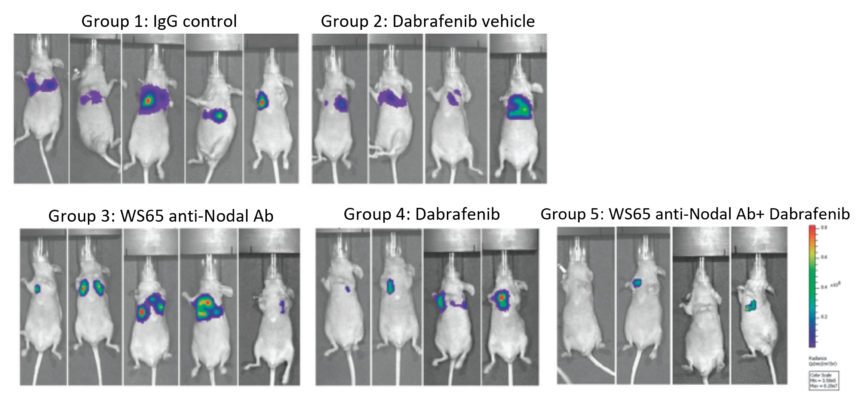
Fig. 6 Anti-Nodal Antibody Synergizes with Dabrafenib (Tafinlar®) in Melanoma
IVIS imaging of Nude mice injected intravenously with the highly metastatic A375SM-L1 human melanoma cells (BRAF V600E mutation). Once tumor formation occurred in lungs, the mice were treated with anti-Nodal antibody or Dabrafenib (Dab) or combination of both over the next 21 days. The combination therapy of Dab plus anti-Nodal antibody was most effective, as shown in Group 5 (upper row-IVIS imaging; lower rows-Nodal IHC staining of mouse lung tissues: control lungs vs. Dab+anti-Nodal antibody treated lungs).
Ref. Hendrix et al., Lab Invest. 2017;97(2):176-186
Fig. 7 Lefty inhibits melanoma tumor growth
Lefty protein is a member of TGF-β super-family and an important cytokine, regulating the embryonic development as well as stem cell differentiation and regulation. Lefty is an antagonist of Nodal, blocks Nodal actions by competing for access to the ligand binding site of Cripto or directly binding to Nodal. Lefty inhibits glioma growth by suppressing Nodal-activated Smad and ERK1/2 pathways.
Ref. Strizzi et al., Expert Rev Dermatol. 2009;4(1):67-78
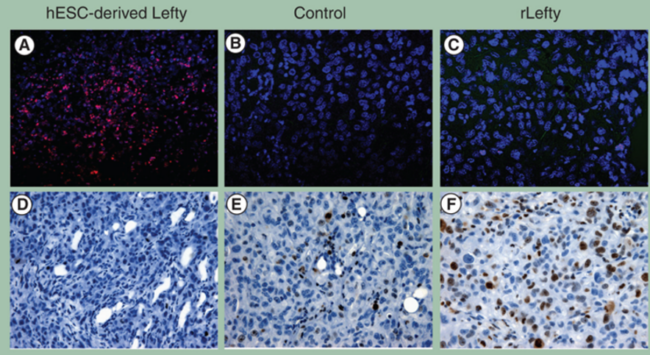
Fig. 8 Affinity purified hESC Lefty inhibits Nodal-positive melanoma tumor
C8161 human cutaneous metastatic melanoma cells were injected orthotopically into a nude mouse model and allowed to form a palpable primary tumor mass for 2 weeks. Subsequently, hESC-derived Lefty (estimated <50 ng), rLefty (30 ng) or carrier minus Lefty (control) was delivered intratumorally over a 2-week period. (A–C) Tumor cell apoptosis was determined by immunohistochemical staining for (TUNEL), which appears red in the color photomicrographs. (D–F) Immunohistochemistry of the Ki67 proliferation marker appears as a brown staining product.
Ref. Strizzi et al., Expert Rev Dermatol. 2009;4(1):67-78
Patent List:
| # | Title | Patent/Application Number | Country |
| 1 | Methods of inhibiting tumor cell aggressiveness using the microenvironment of human embryonic stem cells | 7,666,423 | US* |
| 2 | Methods of inhibiting tumor cell aggressiveness using the microenvironment of human embryonic stem cells | 8,106,004 | US* |
| 3 | Methods of inhibiting tumor cell aggressiveness using the microenvironment of human embryonic stem cells | 8,669,239 | US* |
| 4 | Methods of inhibiting tumor cell aggressiveness using the microenvironment of human embryonic stem cells | 8,975,037 | US* |
| 5 | Methods of inhibiting tumor cell aggressiveness using the microenvironment of human embryonic stem cells | AU2007279205 | AU* |
| 6 | Methods of inhibiting tumor cell aggressiveness using the microenvironment of human embryonic stem cells | JP5693004 | JP* |
| 7 | Anti-nodal antibodies and methods of using same | 9,688,750 | US* |
| 8 | Anti-nodal antibodies and methods of using same | 10,508,147 | US* |
| 9 | Novel anti-nodal antibodies and methods of using same | JP6574257 | JP* |
| 10 | Novel anti-nodal antibodies and methods of using same | EP3204414 | EP* |
| 11 | Anti-nodal antibodies and methods of using same | 10,494,428 | US* |
| 12 | Nodal regulation of cancer drug resistance gene | PCT/US2020/014256 | PCT1 |
| 13 | Suppression of cellular transformation and dysplasia by topical application of Lefty | 10,117,908 | US* |
| 14 | Pharmaceutical composition comprising Lefty | 11,141,459 | US* |
| 15 | Humanized affinity-matured anti-Nodal neutralizing Ab for cancer treatment | Under preparation | |
*Approved patents 1Published
Selected Publications:
- Higher Nodal expression is often associated with poorer survival in patients diagnosed with melanoma and treated with anti-PD1 therapy. Gascard PD, Wang X, Nosrati M, Kim KB, Kashani-Sabet M, Tlsty TD, Leong SP, Hendrix MJC. Pathol Oncol Res. 2024 Sep 23;30:1611889. doi: 10.3389/pore.2024.1611889. eCollection 2024. PMID: 39376672 Free PMC article.
- Heterogeneity of Melanoma with Stem Cell Properties. Seftor EA, Margaryan NV, Seftor REB, Hendrix MJC. Adv Exp Med Biol. 2019;1139:105-114. doi: 10.1007/978-3-030-14366-4_6. PMID: 31134497 Review.
- The Stem Cell Phenotype of Aggressive Breast Cancer Cells. Margaryan NV, Hazard-Jenkins H, Salkeni MA, Smolkin MB, Coad JA, Wen S, Seftor EA, Seftor REB, Hendrix MJC. Cancers (Basel). 2019 Mar 8;11(3):340. doi: 10.3390/cancers11030340. PMID: 30857267 Free PMC article.
- Heterogeneity and Plasticity of Melanoma: Challenges of Current Therapies. Hendrix MJC, Seftor EA, Margaryan NV, Seftor REB. In: Ward WH, Farma JM, editors. Cutaneous Melanoma: Etiology and Therapy [Internet]. Brisbane (AU): Codon Publications; 2017 Dec 21. Chapter 4. PMID: 29461776 Free Books & Documents. Review.
- Targeting the Stem Cell Properties of Adult Breast Cancer Cells: Using Combinatorial Strategies to Overcome Drug Resistance. Margaryan NV, Seftor EA, Seftor REB, Hendrix MJC. Curr Mol Biol Rep. 2017 Sep;3(3):159-164. doi: 10.1007/s40610-017-0067-5. Epub 2017 Jul 10. PMID: 29152453 Free PMC article.
- Nodal Signaling as a Developmental Therapeutics Target in Oncology. Kalyan A, Carneiro BA, Chandra S, Kaplan J, Chae YK, Matsangou M, Hendrix MJC, Giles F. Mol Cancer Ther. 2017 May;16(5):787-792. doi: 10.1158/1535-7163.MCT-16-0215. PMID: 28468864 Review.
- Targeting melanoma with front-line therapy does not abrogate Nodal-expressing tumor cells. Hendrix MJ, Kandela I, Mazar AP, Seftor EA, Seftor RE, Margaryan NV, Strizzi L, Murphy GF, Long GV, Scolyer RA. Lab Invest. 2017 Feb;97(2):176-186. doi: 10.1038/labinvest.2016.107. Epub 2016 Oct 24. PMID: 27775691
- Lefty Glycoproteins in Human Embryonic Stem Cells: Extracellular Delivery Route and Posttranslational Modification in Differentiation. Khalkhali-Ellis Z, Galat V, Galat Y, Gilgur A, Seftor EA, Hendrix MJC. Stem Cells Dev. 2016 Nov 1;25(21):1681-1690. doi: 10.1089/scd.2016.0081. Epub 2016 Sep 19. PMID: 27554431 Free PMC article.
- Translational significance of Nodal, Cripto-1 and Notch4 in adult nevi. Strizzi L, Margaryan NV, Gerami P, Haghighat Z, Harms PW, Madonna G, Botti G, Ascierto PA, Hendrix MJ. Oncol Lett. 2016 Aug;12(2):1349-1354. doi: 10.3892/ol.2016.4755. Epub 2016 Jun 21. PMID: 27446436 Free PMC article.
- Melanocytes Affect Nodal Expression and Signaling in Melanoma Cells: A Lesson from Pediatric Large Congenital Melanocytic Nevi. Margaryan NV, Gilgur A, Seftor EA, Purnell C, Arva NC, Gosain AK, Hendrix MJ, Strizzi L. Int J Mol Sci. 2016 Mar 22;17(3):418. doi: 10.3390/ijms17030418. PMID: 27011171 Free PMC article.
- Nodal expression in triple-negative breast cancer: Cellular effects of its inhibition following doxorubicin treatment. Bodenstine TM, Chandler GS, Reed DW, Margaryan NV, Gilgur A, Atkinson J, Ahmed N, Hyser M, Seftor EA, Strizzi L, Hendrix MJ. Cell Cycle. 2016 May 2;15(9):1295-302. doi: 10.1080/15384101.2016.1160981. PMID: 27007464 Free PMC article.
- Plasticity underlies tumor progression: role of Nodal signaling. Bodenstine TM, Chandler GS, Seftor RE, Seftor EA, Hendrix MJ. Cancer Metastasis Rev. 2016 Mar;35(1):21-39. doi: 10.1007/s10555-016-9605-5. PMID: 26951550 Free PMC article.
- Tumor cell vascular mimicry: Novel targeting opportunity in melanoma. Hendrix MJ, Seftor EA, Seftor RE, Chao JT, Chien DS, Chu YW. Pharmacol Ther. 2016 Mar;159:83-92. doi: 10.1016/j.pharmthera.2016.01.006. Epub 2016 Jan 22. PMID: 26808163 Free PMC article. Review.
- Effects of a novel Nodal-targeting monoclonal antibody in melanoma. Strizzi L, Sandomenico A, Margaryan NV, Focà A, Sanguigno L, Bodenstine TM, Chandler GS, Reed DW, Gilgur A, Seftor EA, Seftor RE, Khalkhali-Ellis Z, Leonardi A, Ruvo M, Hendrix MJ. Oncotarget. 2015 Oct 27;6(33):34071-86. doi: 10.18632/oncotarget.6049. PMID: 26460952 Free PMC article.
- New Anti-Nodal Monoclonal Antibodies Targeting the Nodal Pre-Helix Loop Involved in Cripto-1 Binding. Focà A, Sanguigno L, Focà G, Strizzi L, Iannitti R, Palumbo R, Hendrix MJ, Leonardi A, Ruvo M, Sandomenico A. Int J Mol Sci. 2015 Sep 7;16(9):21342-62. doi: 10.3390/ijms160921342. PMID: 26370966 Free PMC article.
- Cripto-1: an extracellular protein – connecting the sequestered biological dots. Klauzinska M, Bertolette D, Tippireddy S, Strizzi L, Gray PC, Gonzales M, Duroux M, Ruvo M, Wechselberger C, Castro NP, Rangel MC, Focà A, Sandomenico A, Hendrix MJ, Salomon D, Cuttitta F. Connect Tissue Res. 2015;56(5):364-80. doi: 10.3109/03008207.2015.1077239. Epub 2015 Sep 1. PMID: 26327334
- Targeting nodal in conjunction with dacarbazine induces synergistic anticancer effects in metastatic melanoma. Hardy KM, Strizzi L, Margaryan NV, Gupta K, Murphy GF, Scolyer RA, Hendrix MJ. Mol Cancer Res. 2015 Apr;13(4):670-80. doi: 10.1158/1541-7786.MCR-14-0077. Epub 2015 Mar 12. PMID: 25767211 Free PMC article.
- Divergence(s) in nodal signaling between aggressive melanoma and embryonic stem cells. Khalkhali-Ellis Z, Kirschmann DA, Seftor EA, Gilgur A, Bodenstine TM, Hinck AP, Hendrix MJ. Int J Cancer. 2015 Mar 1;136(5):E242-51. doi: 10.1002/ijc.29198. Epub 2014 Sep 29. PMID: 25204799 Free PMC article.
- Nodal signaling promotes a tumorigenic phenotype in human breast cancer. Kirsammer G, Strizzi L, Margaryan NV, Gilgur A, Hyser M, Atkinson J, Kirschmann DA, Seftor EA, Hendrix MJ. Semin Cancer Biol. 2014 Dec;29:40-50. doi: 10.1016/j.semcancer.2014.07.007. Epub 2014 Jul 26. PMID: 25073112 Free PMC article. Review.
- Melanoma tumor cell heterogeneity: a molecular approach to study subpopulations expressing the embryonic morphogen nodal. Seftor EA, Seftor REB, Weldon D, Kirsammer GT, Margaryan NV, Gilgur A, Hendrix MJC. Semin Oncol. 2014 Apr;41(2):259-266. doi: 10.1053/j.seminoncol.2014.02.001. Epub 2014 Feb 7. PMID: 24787297 Free PMC article. Review.
- Age-Dependent Association between Protein Expression of the Embryonic Stem Cell Marker Cripto-1 and Survival of Glioblastoma Patients. Tysnes BB, Satran HA, Mork SJ, Margaryan NV, Eide GE, Petersen K, Strizzi L, Hendrix MJ. Transl Oncol. 2013 Dec 1;6(6):732-41. doi: 10.1593/tlo.13427. eCollection 2013 Dec 1. PMID: 24466376 Free PMC article.
- Potential for the embryonic morphogen Nodal as a prognostic and predictive biomarker in breast cancer. Strizzi L, Hardy KM, Margaryan NV, Hillman DW, Seftor EA, Chen B, Geiger XJ, Thompson EA, Lingle WL, Andorfer CA, Perez EA, Hendrix MJ. Breast Cancer Res. 2012 May 11;14(3):R75. doi: 10.1186/bcr3185. PMID: 22577960 Free PMC article.
- Nodal expression and detection in cancer: experience and challenges. Strizzi L, Hardy KM, Kirschmann DA, Ahrlund-Richter L, Hendrix MJ. Cancer Res. 2012 Apr 15;72(8):1915-20. doi: 10.1158/0008-5472.CAN-11-3419. PMID: 22508696 Free PMC article. Review.
- Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Clin Cancer Res. 2012 May 15;18(10):2726-32. doi: 10.1158/1078-0432.CCR-11-3237. Epub 2012 Apr 2. PMID: 22474319 Free PMC article.
- Reactivation of embryonic nodal signaling is associated with tumor progression and promotes the growth of prostate cancer cells. Lawrence MG, Margaryan NV, Loessner D, Collins A, Kerr KM, Turner M, Seftor EA, Stephens CR, Lai J; APC BioResource; Postovit LM, Clements JA, Hendrix MJ. Prostate. 2011 Aug 1;71(11):1198-209. doi: 10.1002/pros.21335. Epub 2011 Jan 12. PMID: 21656830 Free PMC article.
- Embryonic signaling in melanoma: potential for diagnosis and therapy. Strizzi L, Hardy KM, Kirsammer GT, Gerami P, Hendrix MJ. Lab Invest. 2011 Jun;91(6):819-24. doi: 10.1038/labinvest.2011.63. Epub 2011 Apr 4. PMID: 21464823 Free PMC article.
- Regulation of the embryonic morphogen Nodal by Notch4 facilitates manifestation of the aggressive melanoma phenotype. Hardy KM, Kirschmann DA, Seftor EA, Margaryan NV, Postovit LM, Strizzi L, Hendrix MJ. Cancer Res. 2010 Dec 15;70(24):10340-50. doi: 10.1158/0008-5472.CAN-10-0705. PMID: 21159651 Free PMC article.
- Cancer hallmarks in induced pluripotent cells: new insights. Malchenko S, Galat V, Seftor EA, Vanin EF, Costa FF, Seftor RE, Soares MB, Hendrix MJ. J Cell Physiol. 2010 Nov;225(2):390-3. doi: 10.1002/jcp.22280. PMID: 20568225 Free PMC article.
- Epigenetically reprogramming metastatic tumor cells with an embryonic microenvironment. Costa FF, Seftor EA, Bischof JM, Kirschmann DA, Strizzi L, Arndt K, Bonaldo Mde F, Soares MB, Hendrix MJ. Epigenomics. 2009 Dec;1(2):387-98. doi: 10.2217/epi.09.25. PMID: 20495621 Free PMC article.
- Nodal as a biomarker for melanoma progression and a new therapeutic target for clinical intervention. Strizzi L, Postovit LM, Margaryan NV, Lipavsky A, Gadiot J, Blank C, Seftor RE, Seftor EA, Hendrix MJ. Expert Rev Dermatol. 2009;4(1):67-78. doi: 10.1586/17469872.4.1.67. PMID: 19885369 Free PMC article.
- Development and cancer: at the crossroads of Nodal and Notch signaling. Strizzi L, Hardy KM, Seftor EA, Costa FF, Kirschmann DA, Seftor RE, Postovit LM, Hendrix MJ. Cancer Res. 2009 Sep 15;69(18):7131-4. doi: 10.1158/0008-5472.CAN-09-1199. Epub 2009 Sep 8. PMID: 19738053 Free PMC article. Review.
- The epigenetic influence of tumor and embryonic microenvironments: how different are they? Abbott DE, Bailey CM, Postovit LM, Seftor EA, Margaryan N, Seftor RE, Hendrix MJ. Cancer Microenviron. 2008 Dec;1(1):13-21. doi: 10.1007/s12307-008-0004-5. Epub 2008 Feb 20. PMID: 19308681 Free PMC article.
- Emerging roles of nodal and Cripto-1: from embryogenesis to breast cancer progression. Strizzi L, Postovit LM, Margaryan NV, Seftor EA, Abbott DE, Seftor RE, Salomon DS, Hendrix MJ. Breast Dis. 2008;29:91-103. doi: 10.3233/bd-2008-29110. PMID: 19029628 Free PMC article.
- Potential for cripto-1 in defining stem cell-like characteristics in human malignant melanoma. Strizzi L, Abbott DE, Salomon DS, Hendrix MJ. Cell Cycle. 2008 Jul 1;7(13):1931-5. doi: 10.4161/cc.7.13.6236. Epub 2008 May 5. PMID: 18604175 Free PMC article.
- Role of nodal signaling and the microenvironment underlying melanoma plasticity. Postovit LM, Margaryan NV, Seftor EA, Hendrix MJ. Pigment Cell Melanoma Res. 2008 Jun;21(3):348-57. doi: 10.1111/j.1755-148X.2008.00463.x. Epub 2008 Apr 26. PMID: 18444961 Review.
- Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor RE, Hendrix MJ. Proc Natl Acad Sci U S A. 2008 Mar 18;105(11):4329-34. doi: 10.1073/pnas.0800467105. Epub 2008 Mar 11. PMID: 18334633 Free PMC article.
- Reprogramming metastatic tumour cells with embryonic microenvironments. Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM. Nat Rev Cancer. 2007 Apr;7(4):246-55. doi: 10.1038/nrc2108. PMID: 17384580 Review.
- Targeting Nodal in malignant melanoma cells. Postovit LM, Seftor EA, Seftor RE, Hendrix MJ. Expert Opin Ther Targets. 2007 Apr;11(4):497-505. doi: 10.1517/14728222.11.4.497. PMID: 17373879 Review.
- The commonality of plasticity underlying multipotent tumor cells and embryonic stem cells. Postovit LM, Costa FF, Bischof JM, Seftor EA, Wen B, Seftor RE, Feinberg AP, Soares MB, Hendrix MJ. J Cell Biochem. 2007 Jul 1;101(4):908-17. doi: 10.1002/jcb.21227. PMID: 17177292 Review.
- Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Nat Med. 2006 Aug;12(8):925-32. doi: 10.1038/nm1448. Epub 2006 Jul 30. PMID: 16892036